Scientists reveal a gating mechanism of the ECF-type ABC transporters
Energy-coupling factor (ECF) transporters are a large family of novel ATP-binding cassette (ABC) transporters responsible for micronutrients uptake in plants and bacteria. Each ECF transporter is constituted by an energy-coupling module (ECF module) consisting of a transmembrane T protein (EcfT) and two nucleotide-binding proteins EcfA and EcfA’, and another transmembrane substrate-specific binding protein EcfS. A number of structural studies have been carried out focusing on the transport mechanism of ECF transporters including those from Dr. Zhang Peng’s group at the Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences/CAS. In the previous studies, they solved the first holotransporter structure of ECF transporters-the folate ECF transporter, which revealed the architecture and intramolecular interactions (Xu et al. Nature, 2013). Then by determining a pantothenate transporter structure, the molecular basis underlying ECF module sharing was disclosed (Zhang et al. PNAS, 2014). These studies led to a hypothetical working model of ECF transporters (Zhang et al. Trends Microbiol, 2013).
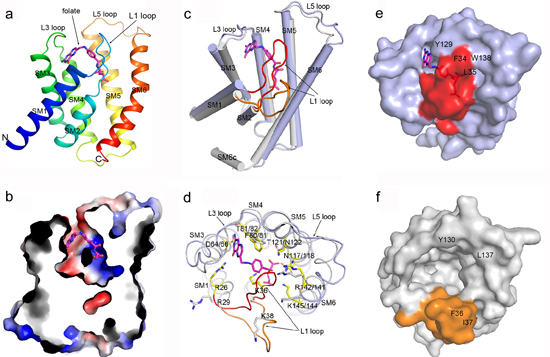
Currently, the conformations of ECF transporters obtained are limited, structures of a single S component crystallized both in substrate bound and free conformations are needed for a deeper understanding of the ECF transporters. In a recent study, researchers from Dr. ZHANG Peng’s group solved the structure of the EcfS protein of a folate ECF transporter EfFolT bound with its substrate folate. Structural and biochemical analyses reveal the residues constituting the folate binding pocket and determining the substrate binding specificity. Structural comparison of the folate-bound EfFolT with the folate-free LbFolT contained in the holotransporter complex discloses significant conformational change at the L1 loop, and reveals a gating mechanism of ECF transporters in which the L1 loop of EcfS acts as a gate in the substrate binding and release.
AUTHOR CONTACT:
Zhang Peng
National Key Laboratory of Plant Molecular Genetics
Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, CAS.
Tel: 86-21-54924219;
Email:
pengzhang01@sibs.ac.cnFigure. (a) Overall structure of EfFolT in ribbon cartoon, the bound substrate folate is shown as a stick model colored in magenta. (b) A cross-section view shows the folate binding site. (c) Superimposition of folate-bound EfFolT (light blue cylinders, L1 loop colored red is in close state) with substrate-free LbFolT (gray cylinders, L1 loop colored orange is in open state) shows the conformational difference. (d) Top view of (c) shows the conformational differences of the residues constituting the folate binding site. (e)-(f) Top views of EfFolT and LbFolT surface show the difference of the folate binding site.