Study reveals the structure and mechanism of a pantothenate ECF transporter
Energy-coupling factor (ECF) transporters are a group of novel ATP-binding cassette (ABC) transporters responsible for micronutrients uptake in plants and bacteria. Each ECF transporter is constituted by an energy-coupling module (ECF module) consisting of a transmembrane T protein (EcfT) and two nucleotide-binding proteins EcfA and EcfA’, and another transmembrane substrate-specific binding protein EcfS. The structure and transport mechanism of ECF transporters remain largely unknown.
Previous study of Dr. Peng Zhang’s group at the Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences/CAS, reported the structure of a folate ECF transporter, which gave a first view of the architecture of the ECF transporter and revealed the inter-molecular interactions of the four proteins forming the complex; and a working model was proposed based on the structural information. (Xu et al. Nature, 2013; Zhang et al. Trends Microbiol, 2013).
One unique feature of ECF transporters is that different EcfS proteins of group II ECF transporters can share one ECF module. However, the underlying mechanism is not clear until this work. In this study, Peng Zhang’s group determined the structure of a group II ECF transporter–pantothenate transporter from Lactobacillus brevis–LbECF-PanT, which shares the ECF module with the folate/hydroxymethylpyrimidine transporters, LbECF-FolT and LbECF-HmpT. Structural and mutational analyses revealed the residues responsible for pantothenate specific binding. More importantly, they found that although the three EcfS proteins, PanT, FolT and HmpT, are dissimilar in sequences, they use a common surface area composed of the transmembrane helices 1/2/6 (SM1/2/6) to interact with the coupling helices 2/3 (CH2/3) of the same EcfT. In addition, the structure of EcfT is found conformationally dynamic which renders its function as a scaffold to mediate the interaction of the ECF module with different EcfS proteins to form different transporter complexes.
This study entitled “Structure of a pantothenate transporter and implications for ECF module sharing and energy coupling of group II ECF transporters” has been published online in PNAS on Dec 15. This work was funded by the National Natural Science Foundation of China, Ministry of Science & Technology of China, Chinese Academy of Sciences and Science & Technology Commission of Shanghai Municipality. Data were collected at BL17U of the Shanghai Synchrotron Radiation Facility (SSRF).
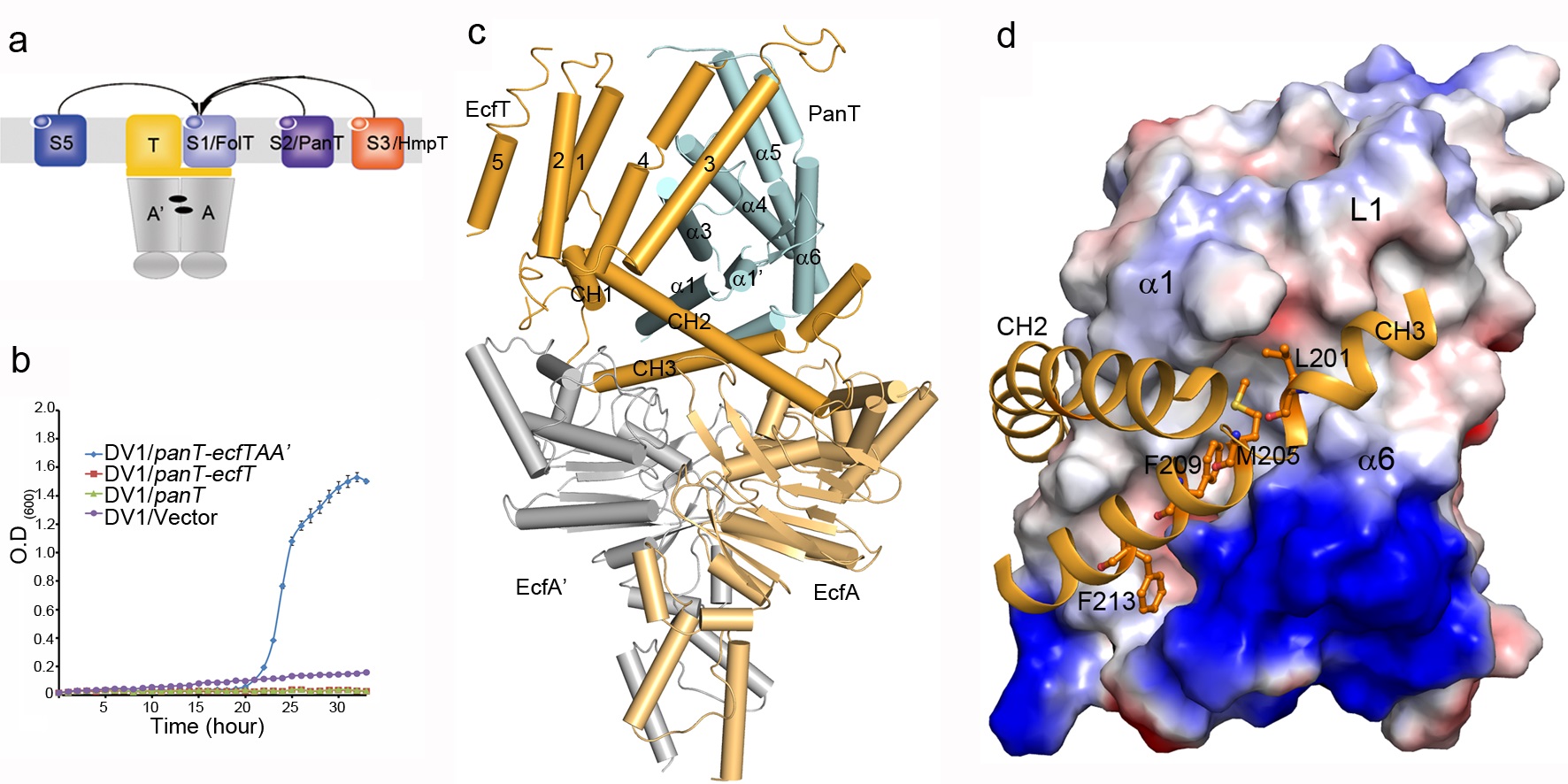
Figure: a, Different EcfS proteins of group-II ECF transporters share one set of ECF module; b, LbECF-PanT is able to complement the growth failure of E.coli DV-1 strain; c, Overall structure of pantothenate ECF transporter, LbECF-PanT; d, Different EcfS proteins use a common surface area composed of the transmembrane helices 1/2/6 (SM1/2/6) to interact with the coupling helices 2/3 (CH2/3) of the same EcfT.
